Share
Pin
Tweet
Send
Share
Send
Phosphor - a substance capable of converting the energy absorbed by it into light radiation. The color of the glow can be different and depends on the filter applied to the surface of the phosphor or its impurities. Photo phosphor is a powder that has the property of glowing in the dark after exposure to natural or artificial light.
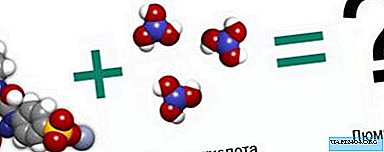
For the manufacture of the phosphor we need coniferous concentrate and boric acid.

We need to buy in the pharmacy not "coniferous extract", but rather "coniferous concentrate", because there is a bright yellow dye tartrazine (E102). The upper pair of blue balls in its molecule is the chromophore (capable of receiving and emitting light) group -N = N- of two nitrogen atoms connected by a double bond. This ability is due to the fact that the fragment -N = N- can be in two positions and the transition energy between them is absorbed / released in the form of light.
In addition, this group is connected on the one hand with a benzene ring of six carbon atoms, and on the other hand with a nitrogen-carbon and another benzene ring. This chain is a kind of corridor in which electrons can "run". Allowable energies of such a run and
determine the color of the radiation.
Despite the fact that we understand how the dye molecule works, it is not yet clear how it forms a phosphor with a boric acid - a molecular photoaccumulator. Do it yourself and experiment.
Pour (or pour if you bought a liquid) in a glass of coniferous concentrate.

Pour a little water - to get an aqueous solution of tartrazine.

Pour boric acid into a spoon

Wet with dye solution.

Stir to wet all the acid

We boil to such a state. We pierce the resulting bubbles with something sharp to ensure good warming of the entire mixture.

Cool, add another dye solution and boil the melt again. It will produce a uniform yellow substance.

This is a phosphor! Use the flash on it:

You can grind it into powder and apply it somewhere, add it to other substances and even to water.
The melting of boric acid with solutions of other dyes - rhodamine and paste from blue gel pens also produced a phosphor, but much worse quality. The big disadvantage of this method of preparing the phosphor is the very short duration of the glow.
Also this phosphor Glows well in ultraviolet light.
Share
Pin
Tweet
Send
Share
Send











